Inflammation, Nutritional Ketosis, and Keto-Immune Modulation: New Insights Into How Virta Can Reverse Type 2 Diabetes

Snapshot: Type 2 diabetes, along with other chronic diseases, is now understood to be due, in part, to over-activity of the otherwise normal processes in the body called inflammation¹. All pharmaceuticals that have been developed to reduce inflammation have side effects, and it seems that the more potent the drug, the more dangerous the side effects. Over the last decade, our research has demonstrated that nutritional ketosis is surprisingly effective at reducing chronic inflammation. This observation helps to explain the prompt and dramatic benefits seen with type 2 diabetes and may also demonstrate the potential to improve a number of other chronic diseases.
An Overview of Inflammation and Nutritional Ketosis
Inflammation is a complex fabric of signals and cellular responses from our immune system that enables our bodies to recognize and respond to infection and injury. Having too weak of an inflammatory response leaves us prone to infection or impaired healing. But having too great of a response, or one that remains over-active for too long, puts us at risk for a form of chronic injury that underlies type 2 diabetes, coronary heart disease, many common cancers, and Alzheimer’s disease.
This balance between too little and too much inflammation is exquisitely delicate, and it is regulated by a number of circumstances including our genetic inheritance, toxins in the environment, and by many components of our diet. One of the first purified drugs designated for human use was an anti-inflammatory compound—aspirin. Currently, we have a host of different drug classes designed to modulate inflammation, but safely managing their dose and duration of use requires professional vigilance to avoid dangerous side effects.
In the past decade, nutritional ketosis has emerged as a potent modulator of inflammation. And, unlike drugs that typically target just one aspect of the body’s immune response, keto-immuno-modulation (KIM) seems to work evenly to balance the anti-inflammatory effect in a safe, sustainable and surprisingly potent way. Using various blood tests that monitor various inflammatory signals, nutritional ketosis has been shown to reduce these signals to a degree comparable to the most powerful drugs currently available. Importantly, it appears to do so without the serious side effects that characterize most pharmaceuticals. Herein we will present some of the evidence behind KIM as a novel therapy that may allow us to prevent and reverse what are currently regarded as chronic progressive diseases.
Measuring Inflammation Levels
Inflammation has been studied by doctors since the time of the Greeks, but those early observations were for the more severe forms that cause obvious symptoms like redness, fever, pain and swelling.
With the invention of the microscope, scientists discovered white blood cells, and saw that they went up during fever and injury, but then came back down when the illness was over. From this information they identified a ‘normal range’ where the white blood cell (WBC) count of most apparently healthy people resided.
Beginning in the mid-1980s however, pioneering scientists initiated a process that is defining a new normal range for the WBC count. Early reports have linked values that are chronically in the upper half of the ‘old normal’ range with increased risk of heart attack, and this is true even in people with normal cholesterol values²,³. In other words, a biomarker of inflammation that is in a range predicting future disease cannot be considered normal.
But that was just the start. Since then, these high-normal WBC levels as well as another test reflective of inflammation in the body called C-reactive protein (CRP) have been shown to also predict the development of type 2 diabetes⁴, many common forms of cancer⁵,⁶, and probably Alzheimer’s⁷.
Beyond the WBC count and CRP, there are many different cell types, proteins, and lipid-derived molecules that participate in the modulation of inflammation and immune functions. These fall under a number of class names like cytokines, chemokines, eicosanoids, and isoprostanes. While the details of these compounds- origins, functions, and interactions, are way too complex for this overview, we need to understand the breadth and delicacy of this web of interacting bioactive factors. And perhaps most importantly, their modulation either ‘up’ or ‘down’ is a delicate process in order to maintain the body’s essential immune and repair functions.
Complexity of Inflammation at a Glance
The processes within the body that control growth, repair, defense against infection, and recovery from injury are some of the most basic to our survival. And, given that they have had over a billion years to evolve, it is understandable that the interlocking functions of immunity and inflammation are multi-layered and extremely complex. Every organ in the body, from the skin to the intestine, has its own characteristic defense that goes into action when a threat is perceived. When any organ is damaged it releases signals into the circulation to alert the rest of the body. This is how a local infection in a foot or a lung can cause the whole body to develop a fever.
So, whether it is an acute local infection causing major inflammation and fever, or something more subtle, like a dietary allergen irritating the intestine, these systemic immune and inflammatory signals can be measured in the blood. Some of these measurable signals are cell types called white blood cells, some are proteins that are made in different organs like muscle, liver, or adipose tissue, and some are peroxidized fats released from cell membranes. Through these circulating signals, the body’s various systems communicate, and the whole process is elegantly choreographed to protect life and function without over-reacting (e.g., driving fever too high or making way too many white blood cells).
There are circumstances, however, when this delicate balance gets too much stimulation and a class of disorders called auto-immune disease can occur. In this instance, the body’s activated defenses attack some of its own organs, causing conditions like rheumatoid arthritis, lupus, psoriasis, and type 1 diabetes. These immune disorders in turn can increase levels of the circulating inflammatory signals, and over time these can affect additional organs. For example, people with long lasting rheumatoid arthritis are at increased risk of heart disease, as are people with type 2 diabetes.
Drugs that Reduce Inflammation
Starting with aspirin, managing inflammation and immune responses has been a favorite target for drug development. In general, the older, established drugs tend to have more general modes of action and a broader spectrum of side effects. Recent pharmaceutical research has moved to target specific enzymes, bioactive molecules, or white blood cell types involved in inflammation to try to reduce side effects, but this has turned out to be a double-edged sword. By focusing on just one single step in the complex cascade of the inflammation/immune system, there is a strong tendency to distort this system rather than reduce the inflammatory effect in a balanced manner.
Table 1. Examples of anti-inflammatory drugs, their uses, and side effects.
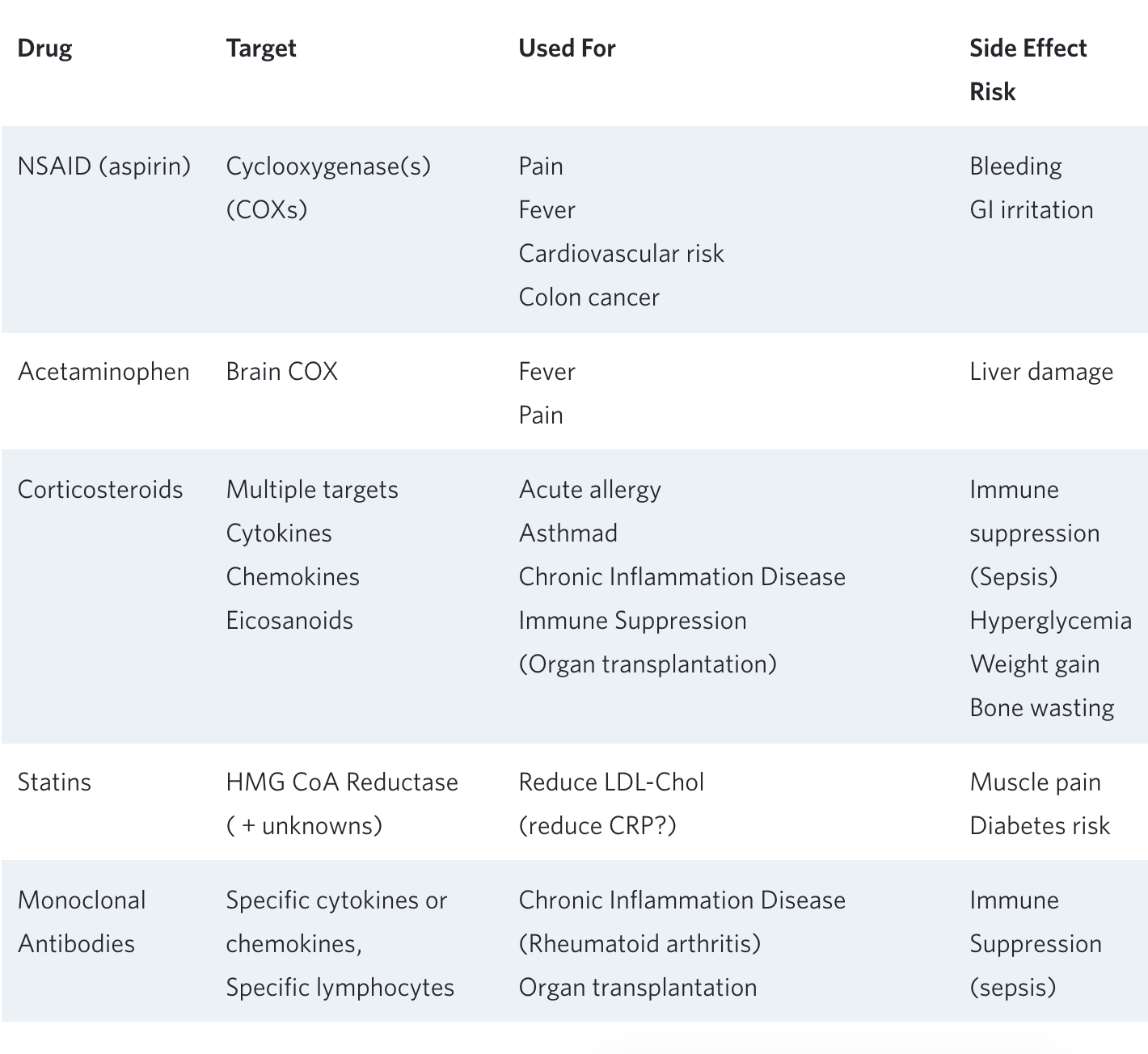
The risks associated with chronic use of a variety of anti-inflammatory drugs are well known and often outweigh the desired benefits. One example is chronic aspirin use, which has been used to reduce heart attacks in people already diagnosed with coronary artery disease. When used in this instance, (as ‘secondary prevention’), it is associated with reduced long-term mortality. However, when aspirin is used routinely in people without known heart disease (primary prevention), overall mortality—primarily due to fatal hemorrhage—is significantly increased⁸.
In another example, a mono-clonal antibody that targets the cytokine IL-1beta and its downstream product C-reactive protein (CRP) was administered to people with known heart disease and elevated blood CRP levels. The purpose of this study was to see if a drug that reduces CRP, without reducing LDL-cholesterol, could prevent heart attacks. After studying 10,000 people over 4 years, mortality from heart disease was significantly reduced by 15%; but death from all causes was no different between the active drug and placebo groups. The reason: the mono-clonal antibody reduced immune function to the extent that an increased number of people died from infections⁹.
These two examples are characteristic of a host of studies indicating that the long-term side-effects of current anti-inflammatory drugs can end up negating their beneficial effects. Thus, this type of chronic treatment has been reserved for patients whose underlying inflammation puts them at high risk—for example rheumatoid arthritis, severe asthma, or organ transplants.
Dietary Anti-inflammatory Treatments
There are a number of foods or purified nutrients that claim to have anti-inflammatory properties, however, most of those claims are based upon shaky, limited, or non-existent human study data. Some of the popular anti-inflammatory nutrients that, when administered as supplements, have not yet stood up to scrutiny are fish oil¹⁰ and green tea¹¹.
Another popular antioxidant with anti-inflammatory properties is vitamin E (in particular the alpha-tocopherol form). It has been used in hundreds of studies with very mixed results. At a very high dose (1200 mg/day) Vitamin E appears to reduce CRP in people with type 2 diabetes¹² but may come with other risks¹³. A different natural form of vitamin E—gamma-tocopherol—has much more potent anti-inflammatory and oxidative stress lowering properties when used alone¹⁴,¹⁵,¹⁶,¹⁷ or in combination with the omega-3 fatty acid DHA¹⁸. This latter study suggests that potent anti-inflammatory effects can be achieved, with apparent safety, by combining moderate doses of nutrients wherein each component acts on a different aspect of the inflammation web.
Aside from supplementation, weight loss itself has been shown to reduce inflammation¹⁹, and it appears that the greater the weight loss the larger the anti-inflammatory effect²⁰. This could be attributable to a reduction in the amount of very inflammatory visceral (belly) fat, and/or a result of some patients being in nutritional ketosis.
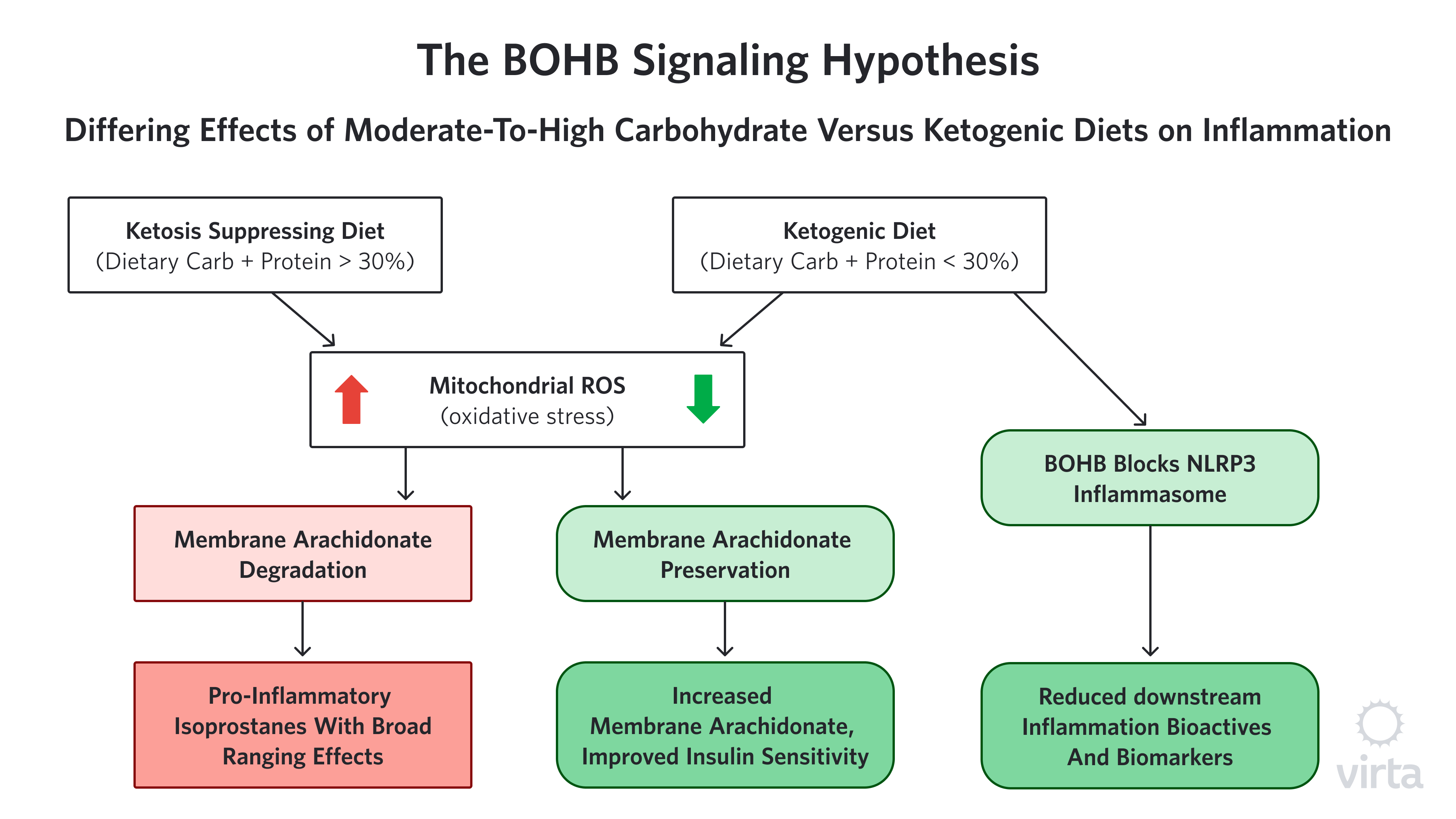
New evidence that BOHB has potent regulatory effects on inflammation
Among the many ‘nutritional factors’ with potential anti-inflammatory properties, the ketone beta-hydroxybutyrate (BOHB) is emerging as both highly potent and uniquely safe as a long-term treatment for inflammation. Long discounted as just a by-product of fat metabolism, BOHB has recently been found to have significant gene signaling/regulating (i.e., epigenetic) effects. In the physiologically normal range that is seen with nutritional ketosis, BOHB activates a number of different genes that protect our cells from oxidative stress²¹ and inflammation²².
We hear a lot about the dangers of free radicals and oxidative stress, but how does oxidative stress cause inflammation? One possible way that reactive oxygen species (ROS), aka ‘free radicals’ appear to be connected to inflammation is through the creation of compounds called isoprostanes. Isoprostanes are created when ROS attack specific fatty acids in cell membranes. These isoprostanes are structurally similar to some of the pro-inflammatory prostaglandins made by cyclo-oxygenase (COX) enzymes—the compounds that are blocked by NSAIDs. But in this case, because there is no enzyme involved in the formation of isoprostanes, NSAIDs can’t block their formation. However, by enhancing the body’s ability to stop ROS before they can attack membrane fatty acids, BOHB prevents this whole class of pro-inflammatory compounds from being created in the first place.
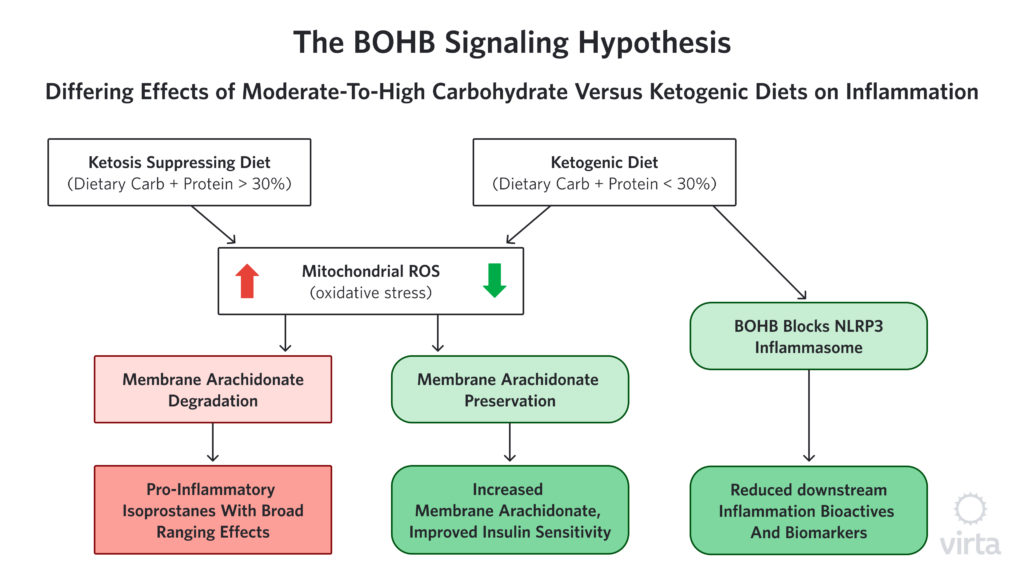
The hypothesis that isoprostane production by ROS is an important contributor to chronic inflammation, and thus influential independent of acute inflammation or injury, is hard to prove. This is because there are many (perhaps dozens) of different isoprostane molecules that result from the random attacks of ROS on membrane fatty acids. Many of these resultant isoprostanes are themselves highly reactive, so they change or disappear quickly, making them difficult to measure accurately. As a result, it is challenging to determine the variance among individuals as well as the influence of factors like a ketogenic diet on isoprostane production.
That said however, we have noticed and reported in multiple human studies that upon starting a well-formulated ketogenic diet, the fatty acid most commonly attacked by ROS, called arachidonic acid (AA), promptly increases²³,²⁴,²⁵; and when the ketogenic diet is stopped, AA quickly goes back to the lower, baseline level²³ (Volk MEAL Study, unpublished). It is important to note that at the same time that we see this ketogenic diet-associated rise in AA, the level of its immediate precursor goes down sharply. A reduction in the precursor provides an indication of how much AA the body is making. These ‘footprints in the sand’ strongly suggest that much less AA is being destroyed by ROS when the body is in nutritional ketosis, therefore less needs to be made in order to maintain optimum membrane levels of this important essential fatty acid. (Figure 1) Intriguingly, the level of AA in muscle membrane is strongly correlated with insulin sensitivity²⁶ thus offering a novel mechanism to explain the heretofore mysteriously prompt improvement in insulin sensitivity upon initiation of a ketogenic diet.
Clinical Studies Demonstrating Reduced Inflammation
These newly discovered actions of BOHB are exciting, but how potent is its anti-inflammatory activity? In a randomized trial comparing two weight loss diets—one ketogenic and the other low fat, high carbohydrate—the ketogenic diet demonstrated much greater anti-inflammatory effects after 12 weeks; and these reductions were seen in multiple inflammation biomarkers, implying a broad-spectrum rather than focused mechanism of action²⁴. Additionally, in our Virta/IUH study of patients with type 2 diabetes, both WBC count and CRP were dramatically reduced in the ketogenic diet group compared to the usual care group at 1 and 2-year follow-up²⁷,²⁸. In particular, the reduction in CRP in the ketogenic diet group at 1 year was comparable in magnitude (35-40%) to what is seen with the most potent statin drug. But unlike the statin, which appears to be primarily focused on CRP and has no effect on WBC count, nutritional ketosis addresses both, providing a more balanced effect on the network of interacting bioactive components influencing inflammation.
Summary: Nutritional Ketosis for Chronic Inflammatory Diseases
Nutritional ketosis has anti-inflammatory and immune-modulating effects that are as potent as the most powerful drugs, but with a more balanced effect across the complex spectrum of bioactive compounds and physiological functions. This explains in part the unique initial, as well as lasting effects, of a well-formulated ketogenic diet in reversing type 2 diabetes, pre-diabetes, and metabolic syndrome.
In addition to improved insulin sensitivity associated with less membrane damage from ROS, the anti-inflammatory properties of nutritional ketosis could be beneficial in other diseases or conditions beyond type 2 diabetes. There are an increasing number of case reports describing improvements in auto-immune diseases such as rheumatoid arthritis and psoriasis, as well as improvements in conditions like polycystic ovary syndrome²⁹,³⁰. These present additional opportunities to understand the underlying processes of certain chronic diseases and explore the potential for potent and sustainable non-pharmaceutical treatment options.
And finally, for almost a century, measurable ketones in one’s blood or urine was considered abnormal. But as we see more and more benefits associated with blood BOHB levels in the range identified in nutritional ketosis, one has to wonder which metabolic state is most beneficial—BOHB of 0.1 to 0.2, or 1.0 to 2.0—particularly in individuals with type 2 diabetes or metabolic syndrome. For example, is someone who is eating a ketosis-suppressing diet containing 300 g/d of ‘healthy carbohydrates’ and exercising 5 hours per week, but still has metabolic syndrome, in a “normal” metabolic state? From an alternative perspective, since reversal of metabolic dysfunction—including metabolic syndrome or type 2 diabetes—can be achieved by eating protein in moderation and total carbohydrate under 50 g/day, perhaps nutritional ketosis should be considered the new metabolic normal for people with diseases associated with or caused by chronic inflammation.
The information we provide at virtahealth.com and blog.virtahealth.com is not medical advice, nor is it intended to replace a consultation with a medical professional. Please inform your physician of any changes you make to your diet or lifestyle and discuss these changes with them. If you have questions or concerns about any medical conditions you may have, please contact your physician.
This blog is intended for informational purposes only and is not meant to be a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a medical condition or any advice relating to your health. View full disclaimer
Are you living with type 2 diabetes, prediabetes, or unwanted weight?

- Odegaard, AO, Jacobs Jr., DR, Sanchez, OA, et al. Oxidative stress, inflammation, endothelial dysfunction and incidence of type 2 diabetes. Cardiovascular Diabetology. 2016; 15:51
- Yarnell JW, Baker IA, Sweetnam PM, et. al. Fibrinogen, viscosity, and white blood cell count are major risk factors for ischemic heart disease. The Caerphilly and Speedwell collaborative heart disease studies. Circulation. 1991; 83(3):836-44
- Kannel WB, Anderson K, Wilson PW. White blood cell count and cardiovascular disease. Insights from the Framingham Study. JAMA. 1992; 267(9):1253-6
- Donath, MY, Shoelson, SE, Type 2 diabetes as an inflammatory disease. Nature Reviews Immunology. 2011;11:98-107
- Hanahan, D., and Weinberg, RA. Hallmarks of Cancer: The Next Generation. Cell.2011; 144:646-67
- Ansar, W., and Ghosh, S. C-reactive protein and the biology of disease. Immunol Res. 2013. 56:131–142
- Van Eldik, LJ., Carrillo, MC., Cole, PE., et al. The roles of inflammation and immune mechanisms in Alzheimer’s disease. Alzheimer’s & Dementia: Translational Research & Clinical Interventions 2016;2: 99-109
- Huang WY, Daugherty E, Shiels, MS, et al. Aspirin Use and Mortality in Two Contemporary US Cohorts. Epidemiology. 2018; 29:126-133
- Ridker PM, Everett BM, Thuren T, et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N Engl J Med 2017; 377:1119-1131
- Muldoon MF., Laderian B., Kuan DC., et. al. Fish oil supplementation does not lower C-reactive protein or interleukin-6 levels in healthy adults. J Intern Med. 2016 Jan;279(1):98-109
- Basu, K., Mei,D.,m Sanchez, K., et al. Green tea minimally affects biomarkers of inflammation in obese subjects with metabolic syndrome. Nutrition. 2011; 27(2): 206-213
- Jialal, I., Devaraj, S., Kaul, N. The Effect of a-Tocopherol on Monocyte Proatherogenic Activity. J. Nutr. 2001; 131: 389S–394S
- Miller III, ER., Pastor-Barriuso,R., Darshan, D., et al. Meta-Analysis: High-Dosage Vitamin E Supplementation May Increase All-Cause Mortality. Ann Intern Med. 2005;142:37-46
- Himmelfarb J., Kane J., Mcmonagle E., et al. Alpha and gamma tocopherol metabolism in healthy subjects and patients with end-stage renal disease. Kidney International. 2003; 64: 978-991
- Chung MY, Yeung SF, Park HJ, et al. Dietary α- and γ-tocopherol supplementation attenuates lipopolysaccharide-induced oxidative stress and inflammatory-related responses in an obese mouse model of nonalcoholic steatohepatitis. J Nutr Biochem. 2010;21(12):1200-6
- Mah E, Noh SK, Ballard KD, et al. Supplementation of a γ-tocopherol-rich mixture of tocopherols in healthy men protects against vascular endothelial dysfunction induced by postprandial hyperglycemia. J Nutr Biochem. 2013;24(1):196-203
- Mah E, Pei R, Guo Y, et al. Greater γ-tocopherol status during acute smoking abstinence with nicotine replacement therapy improved vascular endothelial function by decreasing 8-iso-15(S)-prostaglandin F2α. Exp Biol Med (Maywood). 2015;240(4):527-33
- Himmelfarb J, Phinney S, Ikizler A, et al. Gamma-Tocopherol and Docosahexaenoic Acid Decrease Inflammation in Dialysis Patients. J Renal Nutrition. 2007; 17:296-304
- Sharman MJ, Volek JS. Weight loss leads to reductions in inflammatory biomarkers after a very-low-carbohydrate diet and a low-fat diet in overweight men. Clin Sci (Lond). 2004;107(4):365-9
- Bianchi VE. Weight loss is a critical factor to reduce inflammation. Clin Nutr ESPEN. 2018; 28:21-35.
- Shimazu,T., Hirschey, MD., Newman, J., et al. Suppression of Oxidative Stress by β-Hydroxybutyrate, an Endogenous Histone Deacetylase Inhibitor. Science. 2013;339(6116): 211–214
- Youm, Y-H., Nguyen, KY., Grant, RW., et al. Ketone body β-hydroxybutyrate blocks the NLRP3 inflammasome-mediated inflammatory disease. Nat Med. 2015; 21(3): 263–269
- Phinney SD., Davis PG., Johnson SB., et. al. Obesity and weight loss alter serum polyunsaturated lipids in humans. Am J Clin Nutr. 1991; 53(4):831-8
- Forsythe, CE., Phinney, SD., Fernandez, ML., et al. Lipids. 2008; 43:65–77 Comparison of Low Fat and Low Carbohydrate Diets on Circulating Fatty Acid Composition and Markers of Inflammation. Lipids. 2008; 43:65–77
- Forsythe CE, Phinney SD, Feinman RD, et al, Limited effect of dietary saturated fat on plasma saturated fat in the context of a low carbohydrate diet. Lipids. 2010;45(10):947 62
- Borkman M, Storlien LH, Pan DA, et al. The relation between insulin sensitivity and the fatty-acid composition of skeletal-muscle phospholipids. N Engl J Med. 1993; 328:238-44
- Hallberg, SJ., McKenzie, AL., Williams, PT., et al. Effectiveness and Safety of a Novel Care Model for the Management of Type 2 Diabetes at 1 Year: An Open-Label, Non-Randomized, Controlled Study. Diabetes Ther. 2018; 9:583–612
- Athinarayanan SJ., Adams , RN., Hallberg, SJ.,et al. Long-Term Effects of a Novel Continuous Remote Care Intervention Including Nutritional Ketosis for the Management of Type 2 Diabetes: A 2-year Non-randomized Clinical Trial. 2018. BioRxiv. doi: https://doi.org/10.1101/476275
- Mavropoulos, JC., Yancy, WS., Hepburn, J., et al. The effects of a low-carbohydrate, ketogenic diet on the polycystic ovary syndrome: A pilot study. Nutrition & Metabolism. 2005; 2:35
- Alwahab UA, Pantalone KM, Burguera B. A ketogenic diet may restore fertility in women with polycystic ovary syndrome: a case series. AACE Clinical Case Rep. 2018;4:e427-e431